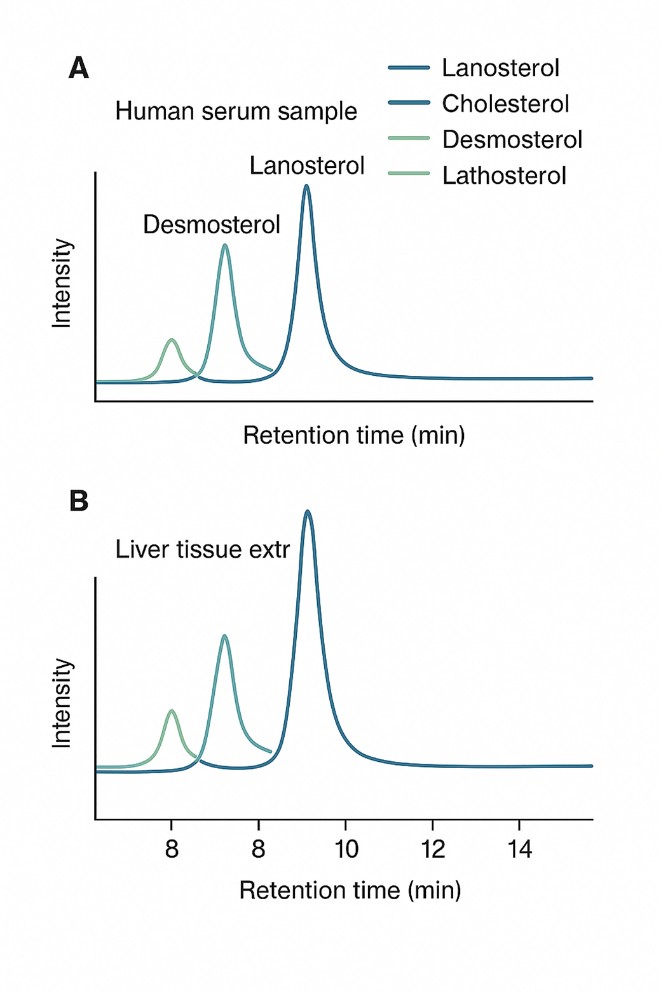
What Is Lanosterol and Why Analyze It?
Lanosterol is the first cyclic sterol produced from squalene in the mevalonate pathway. It sits at the entry point to cholesterol biosynthesis and many downstream sterols.
When lanosterol levels change, it often means:
- The mevalonate pathway is being redirected or blocked.
- Key enzymes in cholesterol synthesis are inhibited or edited.
- Sterol balance in membranes and lipid droplets is shifting.
By quantifying lanosterol together with other sterol intermediates, you can:
- Map bottlenecks in cholesterol biosynthesis.
- Confirm target engagement for sterol-pathway drugs.
- Support flux studies in metabolic engineering projects.
Lanosterol is therefore a sensitive readout for both fundamental sterol biology and mechanism-of-action studies across diverse experimental models.
Lanosterol Analysis Solutions at Creative Proteomics
Creative Proteomics offers lanosterol assays as part of a flexible sterol and lipidomics portfolio. You can choose lanosterol as a stand-alone readout or embed it in broader panels.
Targeted Lanosterol Quantification (Lanosterol Assay)
Use this when you mainly want to see how one intervention changes lanosterol.
- Quantitative lanosterol readout in your chosen matrix
- Suitable for dose–response, time-course, and simple perturbation studies
- Stand-alone lanosterol endpoint when a full sterol panel is not needed
Lanosterol within a Sterol Lipid Panel
Choose this when you need pathway context, not just a single value.
- Lanosterol measured together with cholesterol and key precursors
- Helps locate bottlenecks in cholesterol biosynthesis
- Aligned with our Sterol Lipids Analysis Service
Lanosterol in Mevalonate Pathway Analysis
Select this for broader mevalonate and isoprenoid studies.
Lanosterol Add-On for Lipidomics Studies
Use this when you already run lipidomics and need one key sterol marker.
Analytes Covered by Our Lanosterol and Sterol Panel
Our lanosterol-centered sterol panel includes core precursors, endpoint sterols, phytosterols, and optional oxysterols. The exact combination can be adjusted to your model and matrix.
| Category | Representative analytes* |
|---|
| Lanosterol and precursors | Lanosterol; 24,25-dihydrolanosterol |
| Cholesterol pathway | Zymosterol; lathosterol; desmosterol; 7-dehydrocholesterol (7-DHC) |
| Core sterols | Cholesterol (free / total); ergosterol |
| Phytosterols | Campesterol; β-sitosterol; stigmasterol; brassicasterol |
| Cholesteryl esters | CE 18:1 (cholesteryl oleate); CE 18:2; CE 20:4 and other major cholesteryl esters |
| Optional oxysterols | 24S-, 25-, 27-hydroxycholesterol; 7α-/7β-hydroxycholesterol; 4β-hydroxycholesterol; 7-ketocholesterol; 24,25-epoxycholesterol |
| Optional bile acid–related | Selected primary bile acids and intermediates (on request) |
*The final analyte list is agreed during project setup to match your species, matrix, and research goals.
Why Choose Our Lanosterol Assay?
- High sensitivity
Methods tuned to detect lanosterol and related sterols down to the low-ng/mL range, suitable for limited-volume samples. - Multi-sterol coverage in one run
A single LC–MS/MS run can cover lanosterol plus 10+ late-pathway sterols, reducing sample use and batch effects. - Clean separation of similar sterols
Chromatography optimized to resolve lanosterol from high-abundance cholesterol and structurally close intermediates. - Quantitative, ratio-ready data
Results reported as absolute values and key ratios (e.g., lanosterol/cholesterol, lanosterol/precursors) to support pathway interpretation. - Easy expansion to broader panels
Lanosterol assays can be extended to oxysterols and additional sterols without redesigning the entire analytical workflow.
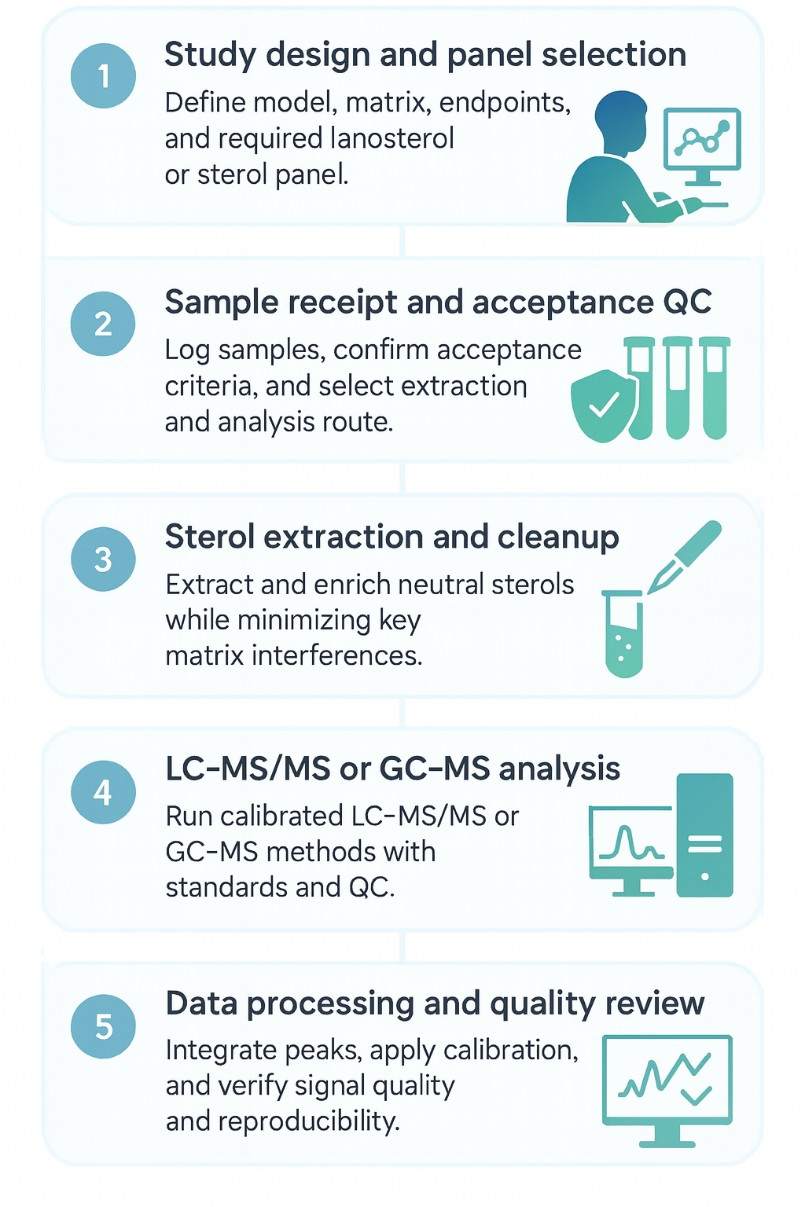
Step-by-Step Workflow for Lanosterol Quantification by LC–MS/MS
Analytical Methods and Core Instruments for Lanosterol Analysis
LC--MS/MS: Primary Platform for Lanosterol Quantification
- UHPLC systems: e.g., Agilent 1290 Infinity II with 2.1 × 100 mm C18 or PFP columns (sub-2 µm) for resolving lanosterol from cholesterol and other sterols.
- Triple-quadrupole MS: e.g., Agilent 6495C using APCI+ or ESI+ and MRM transitions optimized for lanosterol and companion sterols.
- Typical performance: low-ng/mL-level quantification in serum or tissue extracts with a linear dynamic range of ~2–3 orders of magnitude, suitable for dose–response and pathway studies.
HRAM LC-MS for Structural Confirmation (Optional)
- Orbitrap-type HRAM MS: e.g., Q Exactive HF-X or Exploris-class systems for exact-mass confirmation of lanosterol and closely related sterols.
- Resolution: up to ~60,000 FWHM at m/z 200 to support identification of isobaric or co-eluting sterol species and to refine untargeted or semi-targeted workflows.
GC-MS / GC-MS/MS for Derivatized Sterols (Optional)
- GC platforms: e.g., Agilent 7890 GC with 5977-series MS or a triple-quad GC–MS/MS for TMS-derivatized sterols.
- Use cases: legacy datasets, highly hydrophobic matrices, or projects that require direct comparison with existing GC-based sterol methods.

Agilent 6495C Triple Quadrupole (Figure from Agilent)

Agilent 1260 Infinity II HPLC (Fig from Agilent)

Q Exactive HF-X MS

Agilent 7890B-5977A (Figure from Agilent)
Lanosterol Analysis Service: Results and Data Analysis
Standard Deliverables
- Quantitative result tables with lanosterol concentrations, optional co-reported sterols (e.g., cholesterol, precursors), and pre-calculated key ratios.
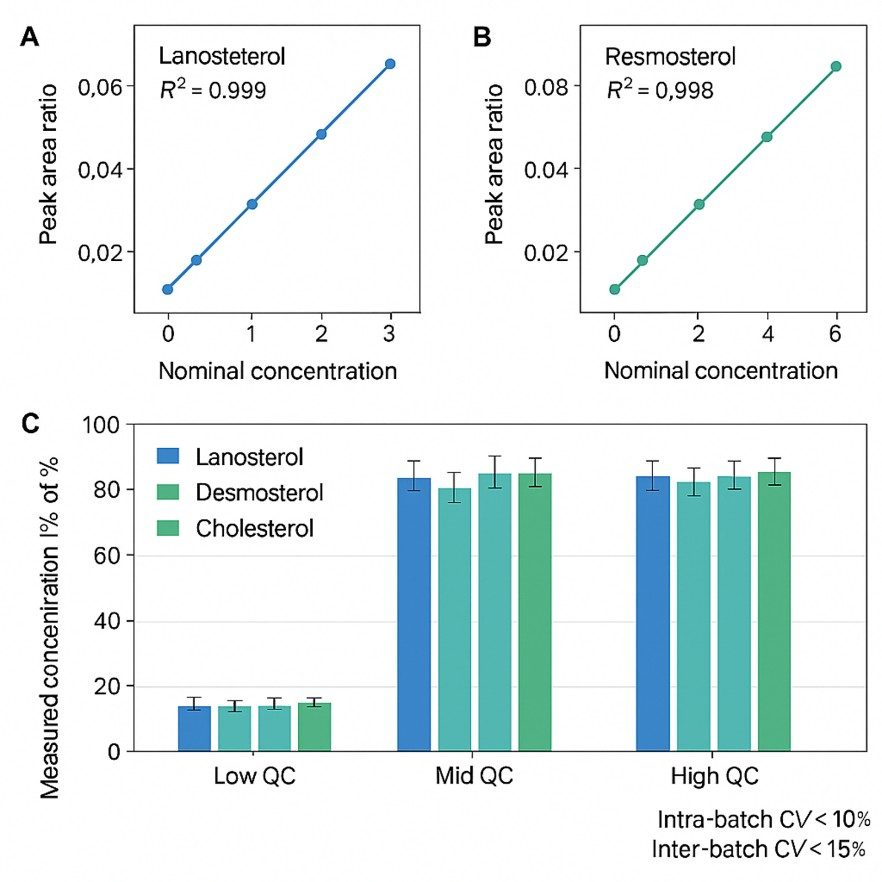
- QC and calibration summary covering calibration range and R², internal/QC sample behavior, and any samples that fail predefined criteria.
- Methods overview describing sample preparation, core LC–MS/MS or GC–MS settings, and the final analyte panel for easy insertion into reports or manuscripts.
Representative chromatograms of lanosterol and related sterols.
Calibration curves and QC performance for the lanosterol sterol panel.
Advanced Data Analysis (Optional)
- Pathway-focused interpretation, linking lanosterol and sterol ratios to specific steps in the mevalonate–cholesterol pathway.
- Group comparison outputs, including fold-change tables, basic statistics, and highlight of the most affected sterol nodes.
- Figure set for presentations, such as bar/box plots, ratio plots, and compact summary charts ready for slides or internal reviews.
Delivery Formats
- Processed data files in Excel or CSV for direct use in your analysis pipelines.
- Integrated PDF report with tables, figures, methods, and QC summary.
- Raw data and peak lists (LC–MS/MS or GC–MS) available on request for in-house reprocessing.
Explore our Lipidomics Solutions brochure to learn more about our comprehensive lipidomics analysis platform.
Download Brochure
Research Applications of Lanosterol Analysis
Mevalonate pathway and cholesterol biosynthesis
Track how genetic or chemical interventions reshape sterol flux and bottlenecks.
Target engagement and mechanism-of-action studies
Use lanosterol and related sterols to read out enzyme inhibition in the pathway.
Metabolic engineering and strain optimization
Tune sterol profiles in yeast, fungi, or engineered cells for improved performance.
Lipidomics and multi-omics integration
Anchor global lipidomics or metabolomics data with a defined sterol pathway node.
Membrane composition and lipid storage
Relate lanosterol changes to membrane sterol balance, lipid droplets, and storage pools.
Preclinical pharmacology and safety assessment
Examine sterol pathway responses to candidate compounds across different experimental models.
Sample Requirements for Lanosterol Analysis Solutions
| Sample type | Recommended amount / volume | Storage and transport | Notes |
|---|
| Serum / plasma | ≥ 100 µL per sample | Freeze at −80 °C; ship on dry ice | Avoid repeated freeze–thaw cycles |
| Whole blood (for plasma) | Follow local collection protocol | Cool immediately; process to plasma quickly | Use anticoagulant compatible with sterol analysis |
| Tissue (e.g., liver, brain) | ≥ 50 mg wet weight | Snap-freeze; store at −80 °C; dry ice ship | Record tissue type and anatomical region |
| Cultured cells | ≥ 1×10⁶ cells per sample | Wash gently; snap-freeze cell pellets | Provide cell line, treatment, and seeding information |
| Yeast / fungal pellets | Equivalent to ≥ 20 mg wet mass | Freeze promptly; store at −80 °C | Indicate species, strain, and growth conditions |
| Microbial cultures | Cell pellet from defined volume | Freeze pellets; avoid preservatives | Discuss media components that may affect extraction |
| Plant material | ≥ 50 mg fresh or frozen tissue | Snap-freeze; keep at −80 °C | Specify tissue type and developmental stage |
| Extracts (organic phase) | ≥ 100 µL extract | Store in suitable solvent at low temperature | Provide solvent composition and extraction protocol |
If your study has strict constraints on sample volume or mass, we can adjust the method where feasible after discussing expected sensitivity and reporting needs.
FAQs for Lanosterol Analysis Service
Why measure lanosterol if I already have total cholesterol data?
Lanosterol sits upstream of cholesterol as the first cyclic sterol in the pathway. Changes in lanosterol (and neighboring intermediates) can reveal where the pathway is slowed or blocked, while total cholesterol alone often stays buffered and hides these bottlenecks.
How many sterols can be quantified in one run, and at what sensitivity?
Modern LC–MS/MS sterol methods routinely quantify about ten late-pathway sterols (including lanosterol, lathosterol, desmosterol, and related species) in a single run using PFP or similar columns. With optimized extraction and MS settings, low-ng/mL concentrations in serum or tissue extracts are achievable, which is sufficient for most preclinical and cell-based projects.
When is a lanosterol-only assay enough, and when do I need a full sterol panel?
A lanosterol-only assay works well when you have a defined target and mainly want a quick, focused readout of whether an intervention perturbs sterol synthesis.
A full sterol panel is preferable when you need to pinpoint which step in the pathway is affected, separate upstream from downstream effects, or link sterol shifts to oxysterols, bile acids, or lipid storage.
How do I choose between LC–MS/MS and GC–MS for lanosterol?
For most projects, LC–MS/MS is the primary choice because it handles complex matrices with shorter run times and no derivatization. GC–MS/GC–MS/MS is useful when you already have legacy GC sterol data, work with very hydrophobic matrices, or specifically need TMS-derivatized sterols to match existing methods.
Can lanosterol analysis be combined with isotope-labeled mevalonate or other tracers?
Yes. Lanosterol sits in the middle of the mevalonate pathway, so it can be measured alongside labeled upstream intermediates or downstream sterols to support flux and mechanism studies. Published work on targeting the mevalonate pathway in cancer and immunity shows how pathway intermediates can act as sensitive pharmacodynamic readouts, and lanosterol fits naturally into this type of design.
How do you deal with matrix effects and structurally similar sterols?
We use sterol-optimized extraction plus C18/PFP chromatography to separate lanosterol from cholesterol and closely related intermediates. MS settings are tuned to specific MRM or exact-mass transitions, and each batch includes calibration and QC samples to monitor interference and signal stability.
Publications
- Asady, B. et al. Quantitative measurement of steroids in Toxoplasma gondii infection. PLOS Pathogens, 2023. https://doi.org/10.1371/journal.ppat.1011566
- Faulstich, N. G. et al. Lipid remodeling supports microbial adaptation in the gut. mBio, 2024.https://doi.org/10.1128/mbio.01059-24
- Sveeggen, T. M. et al. Lipidomic analyses reveal endothelial responses to microenvironmental cues. The FASEB Journal,2023. https://doi.org/10.1096/fj.202201088R
- Gonzalez, M. A. et al. LC–MS-based lipidomics identifies metabolic vulnerabilities in leukemia. Clinical and Translational Medicine, 2022. https://doi.org/10.1002/ctm2.1146
- Gioran, A. et al. Lipidomic profiling links mitochondrial dysfunction to neurodegeneration. EMBO Journal, 2019.https://doi.org/10.15252/embj.201899558