What is Epoxysqualene?
Epoxysqualene is a crucial biochemical compound that acts as an intermediate in the biosynthesis of sterols and steroids. Structurally, it is a cyclic derivative of squalene, an isoprenoid lipid composed of multiple isoprene units. The transformation of squalene into epoxysqualene involves the incorporation of an epoxide group, significantly modifying its chemical properties and biological functions.
This transformation is catalyzed by the enzyme squalene epoxidase, which introduces an oxygen atom into the squalene structure, forming a three-membered epoxide ring. This reaction is a key step in the synthesis of sterols such as cholesterol and steroid hormones. These sterols and hormones are essential for numerous physiological processes, including maintaining membrane fluidity, facilitating cellular signaling, and supporting hormone production.
Beyond its relevance to skin health, epoxysqualene plays a significant role in various biological contexts. Quantitative and qualitative analysis of epoxysqualene is important for several applications:
- Metabolic Pathway Research: Understanding epoxysqualene levels helps elucidate the metabolic pathways involved in sterol and steroid biosynthesis, which is crucial for studying metabolic disorders and the function of these pathways in different tissues.
- Cholesterol and Steroid Synthesis: Analyzing epoxysqualene provides insights into conditions related to cholesterol and steroid synthesis, including certain genetic disorders and endocrine diseases.
- Pharmaceutical Development: In drug research, epoxysqualene analysis can assess the impact of pharmaceutical compounds on metabolic processes, including potential drug interactions and side effects related to sterol and steroid metabolism.
- Environmental and Toxicological Studies: Measuring epoxysqualene levels can reveal how environmental pollutants and toxins affect metabolic processes, aiding in the assessment of chemical exposure effects.
- Cancer Research: Epoxysqualene's involvement in cellular processes makes it relevant for cancer studies, where alterations in its metabolism might be linked to cancer progression or treatment efficacy.
- Nutritional Studies: Evaluating how diet affects epoxysqualene levels can provide insights into the impact of nutrition on sterol and steroid metabolism, relevant for understanding dietary influences on health.
Epoxysqualene Analysis Service by Creative Proteomics
Creative Proteomics provides epoxysqualene analysis service tailored to meet the specific needs of diverse research projects. Our service focuses on both qualitative and quantitative analysis of epoxysqualene to deliver comprehensive insights into its role and behavior in various biological contexts.
Epoxysqualene Quantitative Analysis: Using LC/ESI-MS/MS (Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry), we provide highly accurate and reliable quantification of epoxysqualene levels. This technique allows for the precise measurement of epoxysqualene concentrations in a range of sample types, including skin tissue, cells, and other biological fluids.
Epoxysqualene Qualitative Analysis: We offer detailed qualitative analysis to identify and characterize the presence of epoxysqualene and its metabolic derivatives. This includes profiling the compound's presence in various biological matrices, helping to elucidate its role and significance in different physiological and pathological states.
Epoxysqualene Metabolic Pathway Analysis: Our service includes the analysis of epoxysqualene's involvement in metabolic pathways. By examining its role in sterol and steroid biosynthesis, we provide insights into how epoxysqualene integrates into broader metabolic networks and influences various biological processes.
Epoxysqualene Biomarker Discovery and Validation: We support research aimed at discovering and validating epoxysqualene as a potential biomarker for skin health and disease. This includes evaluating its relevance in conditions such as acne, eczema, psoriasis, dermatitis, and rosacea.
Analytical Techniques for Epoxysqualene Analysis
Creative Proteomics utilizes advanced analytical techniques to ensure precise and reliable measurement of epoxysqualene:
Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry (LC/ESI-MS/MS): This high-resolution technique allows for accurate quantification and identification of epoxysqualene by separating the compound through liquid chromatography and detecting it using mass spectrometry.
Multiple Reaction Monitoring (MRM): Employed within the LC/ESI-MS/MS framework, MRM enhances sensitivity and specificity by monitoring multiple mass transitions for epoxysqualene, ensuring precise detection and quantification.
Deuterated Internal Standards: Used in conjunction with LC/ESI-MS/MS, these standards provide accurate quantification of epoxysqualene by compensating for variability in sample preparation and analysis.
Solid Phase Extraction (SPE): This technique purifies extracts before analysis, removing impurities and concentrating epoxysqualene to improve measurement accuracy.
Sample Requirements for Epoxysqualene Analysis
| Sample Type | Sample Amount | Shipment Conditions |
|---|
| Biological Fluids | 1 mL or more of serum, plasma, or urine | Frozen, on dry ice |
| Tissues | 20 mg of homogenized tissue | Frozen piece of tissue, on dry ice |
| Cell Cultures | 1 × 106 cells or 150 µg of total protein | Frozen, on dry ice |
| Environmental Samples | 1 g of soil or 1 mL of water | Frozen, on dry ice |
| Food Samples | 1 g of homogenized food | Frozen, on dry ice |
If you have any questions about our lipidomics services, please contact us.
Case Regulation of Cholesterol and Coenzyme Q Biosynthesis by Oxidosqualenes
Background
The mevalonate pathway is essential for producing key biomolecules like cholesterol, dolichol, and coenzyme Q (CoQ), all of which have vital physiological roles. Cholesterol is crucial for cell membrane integrity and hormone synthesis, while CoQ is integral to mitochondrial function and antioxidant defense. Dolichol is involved in protein glycosylation.
CoQ levels decrease with age and in certain diseases, leading to conditions like cardiomyopathy and Parkinson's disease. Research has shown that specific epoxidized lipids can both stimulate CoQ synthesis and inhibit cholesterol production, suggesting potential therapeutic strategies for addressing CoQ deficiencies and related metabolic disorders.
Materials & Methods
Reagents
Squalene Epoxidation: Squalene was epoxidized using 3-chloroperoxybenzoic acid in dichloromethane. The epoxides were purified by column chromatography and confirmed by mass spectrometry.
Radioactive Labeling: [3H]Mevalonolactone was synthesized for tracking lipid biosynthesis.
Cell Cultures
HepG2 Cells: Human hepatoblastoma cells were treated with epoxides and [3H]mevalonate to study CoQ9 and CoQ10 synthesis.
Animal Models
Rodent Studies: Male mice and rats were administered squalene epoxides intraperitoneally. Lipid extraction was performed on blood and liver samples for analysis.
Lipid Extraction and Chromatography
Extraction Process: Lipids were extracted using a chloroform:methanol
mixture and analyzed by reversed-phase HPLC and HPTLC.
Chromatography: HPLC and TLC techniques were used to separate and quantify lipids, including squalene epoxides.
Spectroscopic Analysis
Mass Spectrometry (MS): HR-ESI-MS was used for accurate mass determination of epoxides.
Nuclear Magnetic Resonance (NMR) Spectroscopy: 1H NMR confirmed the molecular structure of the epoxides.
Protein Quantification
Bicinchoninic Acid Assay: Protein content was measured using the BCA method.
Statistical Analysis
Data Interpretation: ANOVA followed by Dunnett's test was used to analyze statistical differences, with significance set at p < 0.05.
Results
Structures of Oxidosqualenes and Hydrolytic Products:
The study identified three monoepoxidated squalene derivatives, namely 2,3-oxidosqualene (E1), 6,7-oxidosqualene (E2), and 10,11-oxidosqualene (E3). The hydrolysis of E3 produced three compounds, including E3H1 (with two hydroxyl groups), E3H2 (with one chlorine atom and one hydroxyl group), and E3H3 (with a chlorine atom and hydroxyl group in the opposite orientation). Mass spectrometric analysis confirmed the identities of these compounds.
Impact on Cholesterol, CoQ, and Dolichol Synthesis:
Cholesterol Synthesis: E2 and E3 significantly inhibited cholesterol synthesis in HepG2 cells, with E2 completely blocking it and E3 reducing it by 70% at a 3 μM concentration. The hydrolytic product E3H3 also inhibited cholesterol synthesis, whereas E3H1 and E3H2 showed no effect.
CoQ Synthesis: Interestingly, the same compounds that inhibited cholesterol synthesis (E2, E3, and E3H3) stimulated CoQ synthesis, with CoQ10 and CoQ9 levels increasing notably. In contrast, none of the compounds influenced dolichol synthesis.
Prolonged Treatment Effects:
Cholesterol Reduction: Continuous exposure of HepG2 cells to E3 and E3H3 for 20 days resulted in a 30–35% reduction in cellular cholesterol levels.
CoQ Enhancement: Prolonged treatment also led to a significant increase in CoQ9 and CoQ10 levels, especially CoQ9, which rose by 5- to 8-fold. This suggests a time-dependent elevation in CoQ biosynthesis without affecting mitochondrial respiration or ATP synthesis.
In Vivo Administration:
CoQ Elevation in Mice and Rats: Intraperitoneal injection of E3 in mice led to a 75% increase in plasma CoQ9 levels, with even higher increases observed in apoE-deficient mice and diabetic rats. However, cholesterol levels in both plasma and liver remained unchanged during the treatment period.
Bioavailability: The study confirmed that E3 reaches the bloodstream in a bioactive form capable of inhibiting cholesterol synthesis in white blood cells.
Biosynthesis of CoQ9 and CoQ10:
Substrate Concentration Effects: The study demonstrated that varying mevalonate concentrations affected the CoQ9/CoQ10 ratio, with lower mevalonate levels favoring CoQ9 synthesis. Inhibition of HMG-CoA reductase or squalene synthase altered the biosynthesis pathway, favoring CoQ9 under reduced substrate conditions.
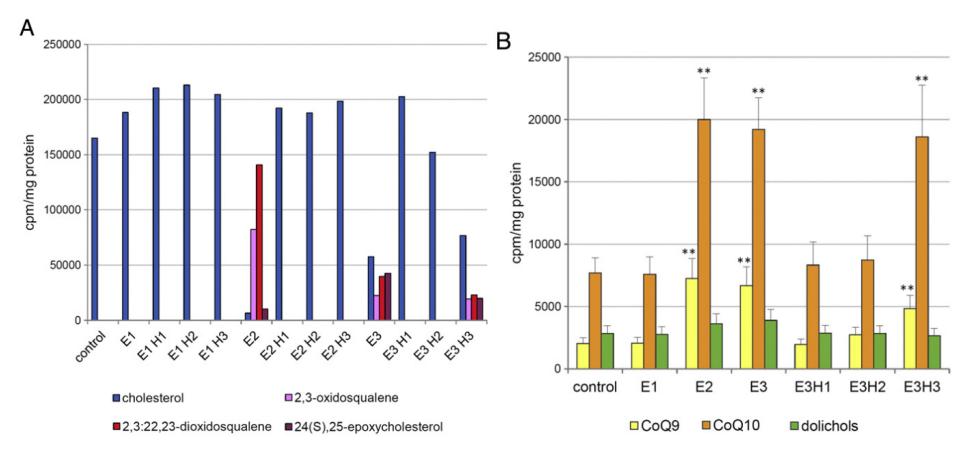 Effects of oxidosqualenes and their hydrolytic products on the synthesis of cholesterol, CoQ and dolichol
Effects of oxidosqualenes and their hydrolytic products on the synthesis of cholesterol, CoQ and dolichol
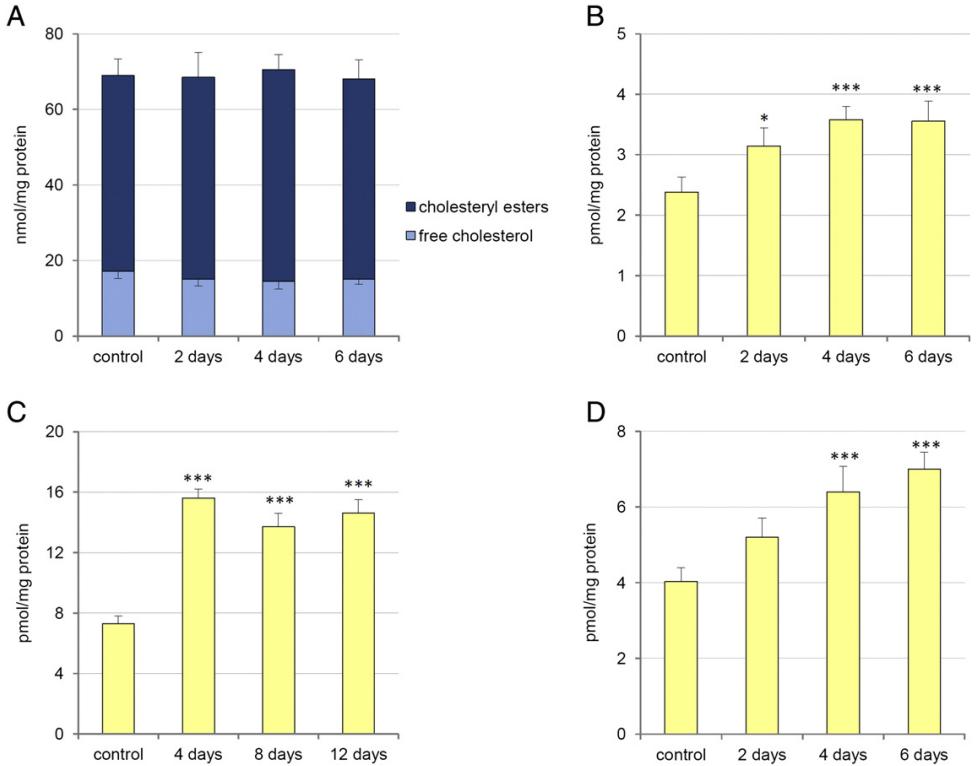 The influence of treatment with 10,11-oxidosqualene on plasma levels of CoQ9 in control mice, apoE deficient mice and GK-rats.
The influence of treatment with 10,11-oxidosqualene on plasma levels of CoQ9 in control mice, apoE deficient mice and GK-rats.
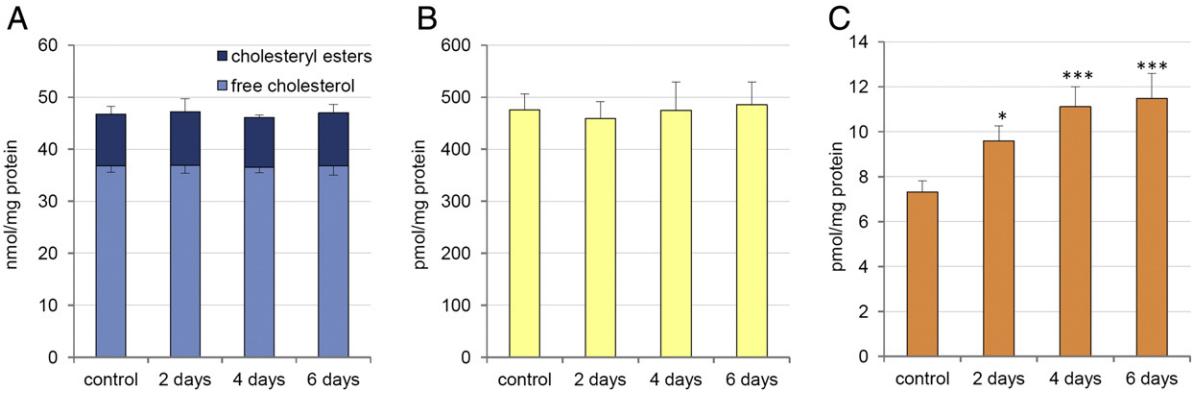 Hepatic levels of cholesterol and CoQ in mice liver treated with 10,11-oxidosqualene
Hepatic levels of cholesterol and CoQ in mice liver treated with 10,11-oxidosqualene
Conclusion
The study reveals that specific oxidosqualenes and their hydrolytic products can modulate the synthesis of cholesterol and CoQ in HepG2 cells and in vivo. While these compounds inhibit cholesterol synthesis, they simultaneously stimulate CoQ production, offering a potential strategy for managing CoQ deficiencies. The findings highlight the complex regulatory mechanisms of lipid homeostasis and suggest that oxidosqualenes could serve as valuable tools in therapeutic contexts, particularly for conditions involving CoQ deficiency. However, the in vivo effects on cholesterol were less pronounced, indicating possible compensatory mechanisms that maintain cholesterol homeostasis. Future research should explore optimizing the delivery and administration of these compounds to enhance their therapeutic potential.
Reference
- Bentinger, Magnus, et al. "Effects of various squalene epoxides on coenzyme Q and cholesterol synthesis." Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids 1841.7 (2014): 977-986.



 Effects of oxidosqualenes and their hydrolytic products on the synthesis of cholesterol, CoQ and dolichol
Effects of oxidosqualenes and their hydrolytic products on the synthesis of cholesterol, CoQ and dolichol The influence of treatment with 10,11-oxidosqualene on plasma levels of CoQ9 in control mice, apoE deficient mice and GK-rats.
The influence of treatment with 10,11-oxidosqualene on plasma levels of CoQ9 in control mice, apoE deficient mice and GK-rats. Hepatic levels of cholesterol and CoQ in mice liver treated with 10,11-oxidosqualene
Hepatic levels of cholesterol and CoQ in mice liver treated with 10,11-oxidosqualene