Free & Total Cholesterol Quantification
Measure both free and total cholesterol to capture baseline and intervention effects across samples.
Our services have earned the trust of companies, schools, and organizations globally, and we remain dedicated to maintaining that trust.
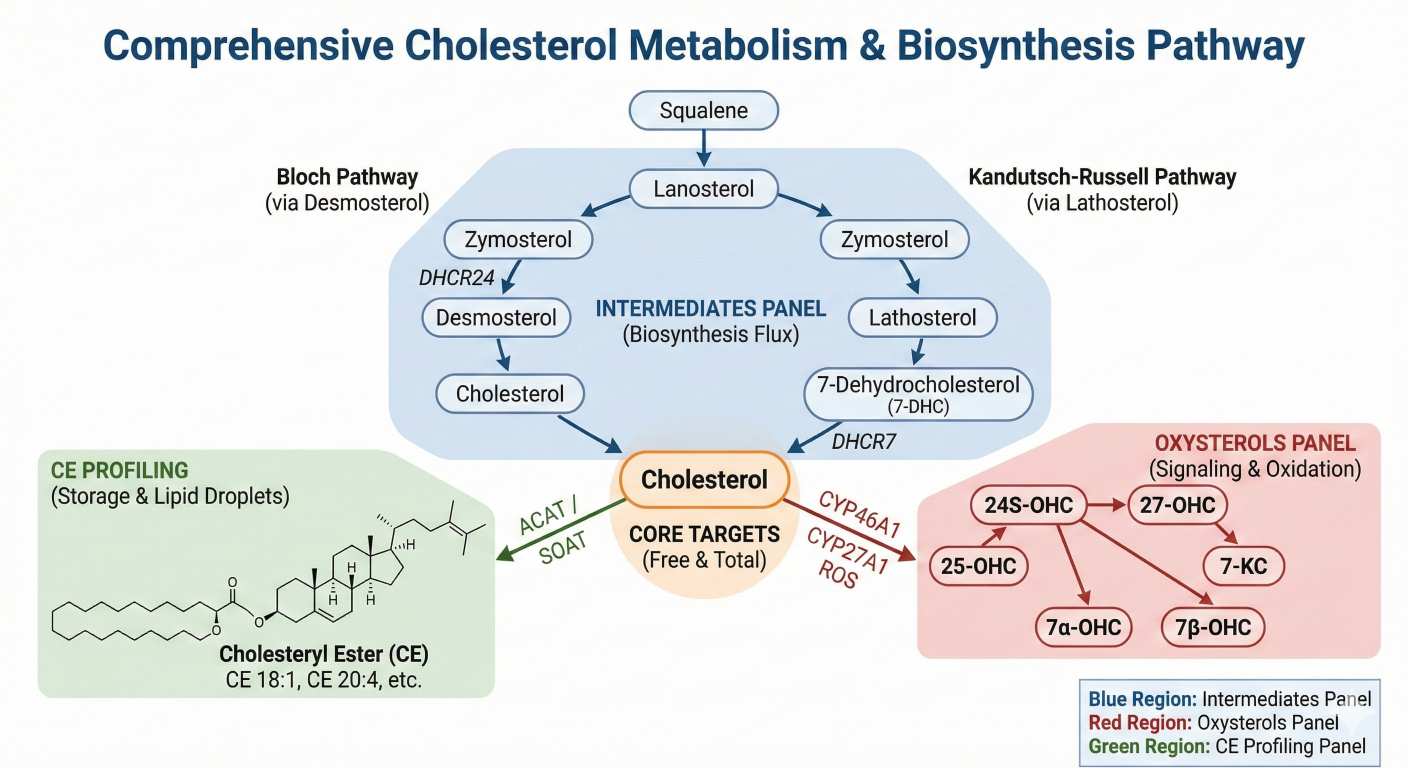
Measuring "cholesterol" alone often answers only whether a change happened—not why it happened. In cholesterol homeostasis research, multiple processes can drive the same endpoint shift:
By combining cholesterol + intermediates + oxysterols/CE, you can build a defensible pathway narrative—especially in drug/genetic perturbation, time-course, and multi-matrix studies.
Free & Total Cholesterol Quantification
Measure both free and total cholesterol to capture baseline and intervention effects across samples.
Cholesterol Biosynthesis Intermediates Panel
Profile key precursors like lanosterol and desmosterol to reveal cholesterol synthesis dynamics and pathway shifts.
Oxysterols Quantification Panel
Quantify signaling-active oxysterols such as 24-OHC and 27-OHC to explore oxidation and lipid regulation pathways.
Cholesteryl Esters (CE) Profiling
Assess CE species to investigate cholesterol storage, esterification, and lipid droplet remodeling.
Not sure which combination fits your study design? Share your matrix, perturbation plan, and targets—our scientists will recommend a pathway-first configuration.
Panels are customizable by matrix and study design (add/remove targets upon request). Target availability and reporting mode may vary by matrix.
| Module | Target Class | Targets (As Listed on This Page) |
|---|---|---|
| Core | Cholesterol | Free cholesterol; Total cholesterol |
| Intermediates | Biosynthesis intermediates | Squalene; Lanosterol; Zymosterol; Desmosterol; Lathosterol; 7-Dehydrocholesterol (7-DHC); 7-Dehydrodesmosterol (optional); Dihydrolanosterol (optional) |
| Oxysterols | Signaling / oxidation-derived sterols | 24S-Hydroxycholesterol (24-OHC); 25-Hydroxycholesterol (25-OHC); 27-Hydroxycholesterol (27-OHC); 7-Ketocholesterol (7-KC); 7α-Hydroxycholesterol (7α-OHC); 7β-Hydroxycholesterol (7β-OHC); 4β-Hydroxycholesterol (4β-OHC) (optional) |
| CE | Cholesteryl esters | CE 14:0; CE 16:0; CE 16:1; CE 18:0; CE 18:1; CE 18:2; CE 18:3; CE 20:3; CE 20:4; CE 20:5; CE 22:5; CE 22:6 (custom expansion available) |
Quick module pairing (research context):
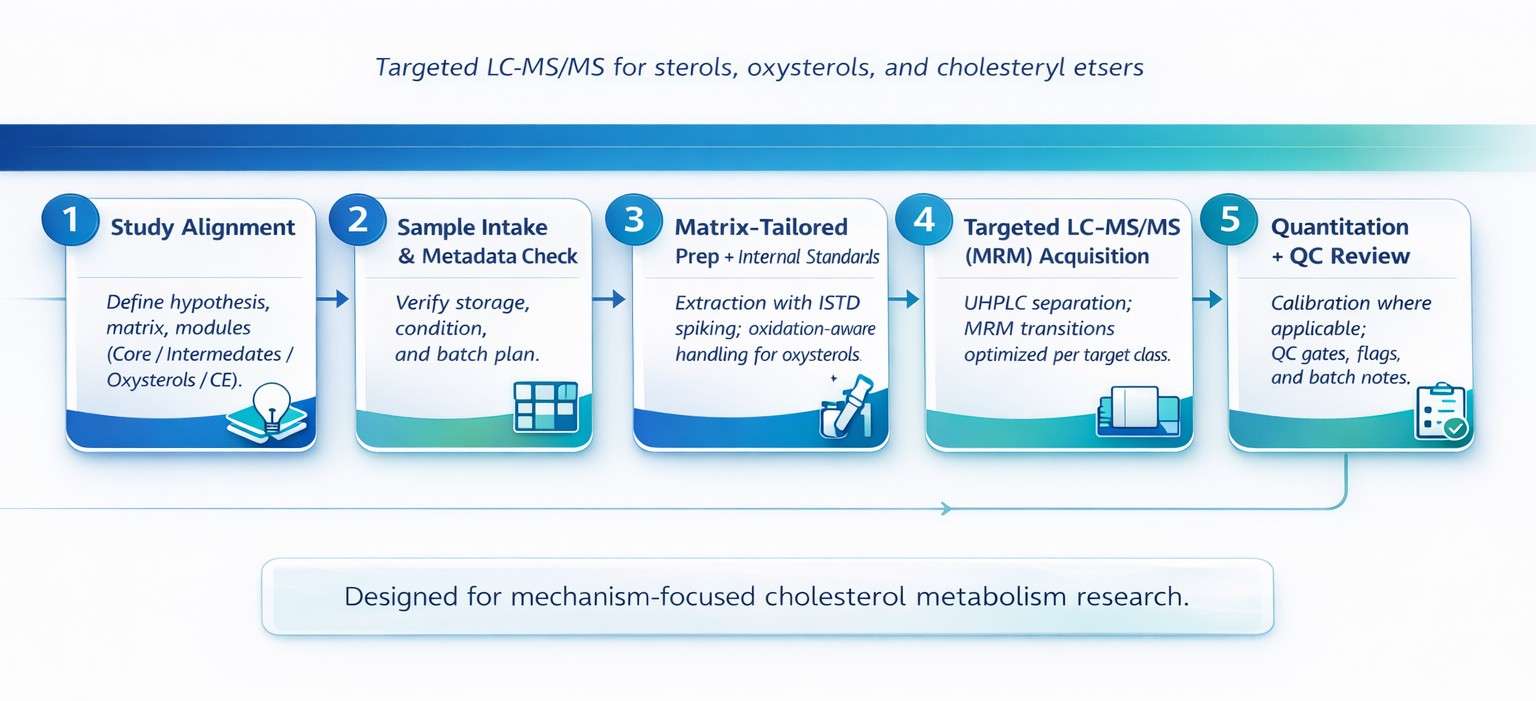
UHPLC gradients are designed for baseline separation of critical sterol isomers (e.g., 7α-OHC vs. 7β-OHC, Desmosterol vs. 7-DHC) to reduce isomeric interference.
High-sensitivity MRM on triple quadrupole platforms (e.g., Agilent 6495C or equivalent) targets low-abundance oxysterols and intermediates with typical pg/mL-level LLOQs (matrix-dependent).
Configured for a 3–4 order quantitation range to cover both high- and low-abundance sterol targets in the same study.
Multi-internal standard strategies—including isotope-labeled sterols where applicable—help correct matrix effects and extraction variability for concentration-based reporting.
Matrix-aware protocols with internal standard monitoring, typically showing 70–130% response/recovery consistency within acceptance criteria (reported in QC summaries where applicable).
QC gating and replicate strategies align with typical precision targets of ≤15% intra-batch CV and ≤20% inter-batch CV (target- and matrix-dependent).
We help plan pathway-oriented studies to reduce rework and improve interpretability across cholesterol biosynthesis, oxysterol signaling, and CE remodeling.
Common Study Formats
If you're unsure what's feasible in your matrix or expected range, contact us for a quick consult.
We support optional platform configurations based on your targets, matrix, and required performance. Below are representative examples—final instrumentation and settings are selected per project.
| Parameter Group | What We Specify (Typical) |
|---|---|
| Chromatography | Column chemistry, mobile phases, gradient program, run time, injection volume |
| MS/MS Acquisition | Polarity mode, MRM strategy, dwell/scan settings (as applicable), collision energy optimization approach |
| Quant Strategy | Calibration approach (when applicable), internal standard correction, data flagging rules |
| Data Processing | Integration rules, QC gates, below-quantitation handling, batch notes |
If your project involves challenging isomers or oxidation-prone targets, we can incorporate additional method considerations (e.g., separation strategy and handling controls) within the method summary.

Agilent 1290 UHPLC System (Figure from Agilent)

Agilent 6495C Triple Quadrupole (Figure from Agilent)
Sterols and oxysterols can be challenging due to isomeric similarity, matrix effects, and oxidation artifacts. We emphasize QC outputs you can verify in the final report.
QC Elements Included (Typical)
Optional Enhancements (Upon Request)
Explore our Lipidomics Solutions brochure to learn more about our comprehensive lipidomics analysis platform.

Metabolic Pathway Mechanisms
Identify biosynthesis vs signaling vs storage-driven changes

Drug Perturbation Studies
Evaluate pathway response to synthesis/efflux/esterification modulators

Genetic Perturbation Experiments
Map sterol node changes after KO/KD/CRISPR edits

Oxidative Stress Biology
Track oxysterol shifts linked to lipid oxidation and signaling

Lipid Droplet And Storage Remodeling
Profile CE remodeling associated with esterification dynamics

Multi-Omics Integration
Link sterol pathway readouts with transcriptomics/proteomics phenotypes
Recommended Sample Amount
| Sample Type | Suggested Amount | Notes |
|---|---|---|
| Plasma / Serum | 50–200 µL | Panel scope and target abundance may change requirements |
| Tissue | 20–100 mg | Specify tissue type and storage conditions |
| Cells | 1–10 million | Provide cell count and processing details |
| Other Matrices | Case-by-case | Bile/feces/microbes and others—please consult before shipping |
Handling and Shipping (Best Practices)
More guidelines?
Why is LC–MS/MS necessary for profiling cholesterol intermediates compared to standard colorimetric kits?
Standard enzymatic kits often lack specificity, significant cross-reacting with structurally similar sterols (e.g., measuring desmosterol as cholesterol). Targeted LC–MS/MS provides absolute specificity through chromatographic separation—crucial for resolving isomers like 7α-OHC versus 7β-OHC—and mass-selective detection (MRM). This ensures accurate quantification of low-abundance precursors that are indistinguishable in bulk assays.
How do you prevent artifactual generation of oxysterols during sample preparation?
Ex vivo oxidation during handling is a critical challenge in oxysterol research. Our protocols minimize artifacts by incorporating antioxidants (e.g., BHT) during extraction, performing steps at controlled temperatures, and strictly limiting exposure to light and air. We verify process integrity using stability-monitored internal standards to ensure results reflect biological status, not preparation artifacts.
What does an elevated ratio of Desmosterol or Lathosterol to Cholesterol indicate biologically?
These ratios serve as markers for enzymatic bottlenecks in biosynthesis. An elevated Desmosterol ratio typically indicates reduced DHCR24 activity (Bloch pathway blockade), while elevated Lathosterol suggests reduced DHCR7 activity (Kandutsch-Russell pathway blockade). Monitoring these ratios is essential for characterizing genetic models or evaluating the distal effects of synthesis inhibitors.
How is "Total Cholesterol" quantified differently from "Free Cholesterol" in this panel?
Free cholesterol is measured directly in the lipid extract. To quantify Total Cholesterol, samples undergo a validated hydrolysis step (chemical or enzymatic, matrix-dependent) to break ester bonds and convert all Cholesteryl Esters (CE) into free cholesterol prior to LC–MS/MS analysis. The esterified fraction is then calculated by the difference.
Why profile individual Cholesteryl Ester (CE) species instead of just measuring total esterified cholesterol?
Measuring total CE mass masks underlying metabolic shifts. Profiling specific CE species based on their attached fatty acid (e.g., CE 18:1 oleate vs. CE 20:4 arachidonate) reveals substrate preference by enzymes like ACAT/SOAT. This provides granular insights into lipid droplet composition and fatty acid flux under conditions like inflammation or atherosclerosis.
Can this service be applied to complex matrices like brain tissue or atherosclerotic plaques?
Yes. We utilize matrix-tailored extraction protocols specifically optimized for lipid-rich and heterogeneous tissues. For brain samples, our methods ensure efficient recovery of brain-specific oxysterols like 24S-OHC. For plaques, we use vigorous homogenization techniques to ensure complete lipid extraction from calcified or fibrotic material.
Do you provide support for interpreting the pathway data beyond raw concentration tables?
Yes. We offer optional advanced analysis to visualize quantitative data within the context of the cholesterol biosynthesis and metabolism pathways. This helps rapidly identify enzymatic bottlenecks—showing upstream precursor accumulation and downstream product depletion—to directly support mechanistic hypotheses in perturbation studies.

Services:
Resource:
Platform:
Online Inquiry
CONTACT US