What are Wax Esters?
Wax esters (WE) belong to the group of fatty esters within the broader category of fatty acyls. Structurally, they consist of a long-chain fatty acid linked to a long-chain fatty alcohol via an ester bond. This characteristic structure allows for variability in the length of both the fatty acid and alcohol components, as well as the presence of multiple double bonds and oxygen-containing substituents such as hydroxy and keto groups.
Wax esters play multifaceted roles in biological systems, ranging from surface coating in plants and insects to energy storage in mammals. Understanding their composition and distribution is crucial for elucidating their diverse biological functions. Analyzing wax esters provides valuable insights into lipid metabolism, ecological interactions, and the development of novel therapeutic interventions.
Wax Esters Analysis Service Projects by Creative Proteomics
Creative Proteomics offers a comprehensive suite of wax esters analysis services, catering to diverse research needs and applications. Leveraging state-of-the-art analytical techniques and expertise in lipidomics, our services enable precise characterization and quantification of wax esters across various sample types.
Targeted Wax Esters Analysis: Utilizing advanced mass spectrometry techniques, such as liquid chromatography-mass spectrometry (LC-MS), we precisely identify and quantify specific molecular species of wax esters in complex biological samples. We employ cutting-edge instrumentation, including the Thermo Scientific Q Exactive series and the SCIEX TripleTOF series, to ensure high sensitivity and accuracy.
Structural Profiling and Molecular Species Identification: Leveraging high-resolution mass spectrometry coupled with liquid chromatography, such as the Agilent 1290 HPLC + 6495 Triplequad system and the Waters ACQUITY UPLC + Xevo TQ-XS system, we provide detailed structural information and identify molecular species of wax esters present in the sample.
Quantitative Analysis of Wax Esters: Our quantitative analysis services enable precise measurement and quantification of wax esters in biological samples. Using state-of-the-art analytical techniques and instrumentation, including the Thermo Scientific TSQ Altis series and the Bruker timsTOF Pro system, we ensure reliable and reproducible results.
Customized Solutions for Wax Esters Analysis: Recognizing the unique research requirements of our clients, we offer customized wax esters analysis solutions tailored to specific experimental needs. From method development to sample preparation optimization, our team collaborates closely with clients to deliver tailored solutions that meet their research objectives effectively.
List of Wax Esters We Can Analyze
| Octyl Palmitate | Cetyl Palmitate | Myristyl Myristate | Stearyl Stearate | Behenyl Behenate |
| Cetyl Stearate | Lauryl Laurate | Myristyl Palmitate | Cetyl Myristate | Stearyl Palmitate |
| Behenyl Palmitate | Stearyl Behenate | Cetyl Behenate | Octyl Stearate | Lauryl Palmitate |
Advanced Technologies for Wax Esters Analysis
Liquid Chromatography-Mass Spectrometry (LC-MS)
LC-MS is a powerful analytical technique combining the separation capabilities of liquid chromatography with the detection and identification capabilities of mass spectrometry. In wax esters analysis, LC-MS enables the precise separation of analytes based on their chemical properties, followed by accurate mass determination and structural elucidation.
Instrumentation: We employ various LC-MS systems, including:
- Agilent 1290 HPLC + 6495 Triplequad system: This high-performance liquid chromatography (HPLC) system coupled with a triple quadrupole mass spectrometer offers unparalleled sensitivity and specificity for wax esters analysis. The precise separation of analytes coupled with advanced mass spectrometry capabilities enables comprehensive characterization and quantification of wax esters with exceptional accuracy.
- Thermo Scientific Q Exactive series: These high-resolution mass spectrometers provide superior mass accuracy and resolution, allowing for precise identification and quantification of wax esters in complex biological samples. The combination of high-resolution mass spectrometry with ultra-high-performance liquid chromatography (UHPLC) ensures robust and reliable analysis of wax esters.
- SCIEX TripleTOF series: Known for its high sensitivity and dynamic range, the TripleTOF series offers excellent performance in wax esters analysis. Coupled with ultra-fast liquid chromatography systems, this platform enables rapid and accurate identification of wax esters, making it ideal for high-throughput lipidomic studies.
Gas Chromatography-Mass Spectrometry (GC-MS)
GC-MS is another powerful analytical technique widely used for wax esters analysis, particularly for volatile and thermally stable compounds. In GC-MS, analytes are separated based on their volatility and then ionized and detected by mass spectrometry, allowing for precise identification and quantification of wax esters.
Instrumentation: Our GC-MS systems include:
- Agilent GC-MS systems: These robust and reliable instruments offer excellent sensitivity and selectivity for wax esters analysis. Equipped with advanced mass spectrometry detectors, such as quadrupole or time-of-flight (TOF) analyzers, these systems provide accurate identification and quantification of wax esters across a wide range of sample matrices.
- Thermo Scientific GC-MS systems: Renowned for their versatility and performance, Thermo Scientific GC-MS systems are widely used in wax esters analysis. With features such as electron ionization (EI) and chemical ionization (CI) sources, as well as various mass analyzers (e.g., quadrupole, ion trap), these systems offer flexibility and reliability in lipidomic profiling.
Nuclear Magnetic Resonance Spectroscopy (NMR)
NMR spectroscopy is a non-destructive analytical technique used for the structural elucidation of organic compounds, including wax esters. In NMR, the nuclei of atoms within the molecule are excited by a magnetic field, producing characteristic signals that can be used to determine the chemical structure and composition of the compound.
Instrumentation: Our NMR systems include:
- Bruker NMR spectrometers: These high-performance instruments offer excellent sensitivity and resolution for wax esters analysis. Equipped with advanced software for data acquisition and analysis, Bruker NMR spectrometers provide detailed structural information, allowing for accurate identification and characterization of wax esters in biological samples.
Sample Requirements for Wax Esters Analysis
| Sample Type | Sample Amount |
|---|
| Sebum Samples | 0.05 - 0.2 g |
| Human Skin Scrapings | 0.1 - 0.5 g |
| Hair Strands | 0.1 - 0.5 g |
| Blood Serum | 0.1 - 0.5 mL |
| Adipose Tissue | 0.1 - 0.5 g |
| Feces | 0.5 - 1 g |
| Urine | 1 - 5 mL |
| Buccal Swabs | 1 - 5 swabs |
If you have any questions about our lipidomics services, please contact us.
Case Carbon Assimilation Pathways in Anaerobic Wax Ester Fermentation of Euglena gracilis Z
Background
Understanding the metabolic pathways involved in wax ester fermentation is crucial for optimizing production in Euglena gracilis Z. Previous research has shown the importance of environmental CO2 fixation in driving this process, particularly under anaerobic conditions.
Samples
Euglena gracilis Z cultures were subjected to anaerobic conditions with varying light exposure, allowing for the investigation of wax ester fermentation and carbon assimilation pathways.
Technical Methods
Cultivation Conditions: E. gracilis Z cultures were grown under normoxic-light conditions for 4 days before switching to hypoxic or anoxic conditions. Light exposure was controlled to assess its impact on wax ester fermentation.
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis: Nonpolar fractions from samples were analyzed using GC-MS to identify and quantify wax esters synthesized under different conditions.
13C-labeling Experiments: Cultures were supplemented with NaH13CO3 and incubated to assess the incorporation of 13C into wax esters. Mass spectral intensities of natural isotopes and 13C-labeled fragments were compared to determine 13C-enrichment levels.
Enzyme Inhibition Studies: The inhibitor 3MPA was used to investigate the role of phosphoenolpyruvate carboxykinase (PEPCK) in CO2 fixation and wax ester biosynthesis.
Results
CO2 Assimilation: External inorganic carbon sources were essential for wax ester fermentation in Euglena gracilis Z under anaerobic-light conditions.
13C-labeling Experiments: 13C-stable isotopes were primarily incorporated into odd-numbered fatty acid moieties of wax esters, indicating the involvement of CO2 assimilation pathways.
Metabolic Pathways: CO2 fixation via PEPCK and the reverse tricarboxylic acid (TCA) cycle played key roles in wax ester biosynthesis.
Enzyme Inhibition Studies: Inhibition of PEPCK activity with 3MPA hindered CO2 assimilation and wax ester fermentation, highlighting the importance of this enzyme in the process.
Future Directions: Further research is needed to optimize substrate supply for anoxic CO2 fixation and enhance wax ester production in Euglena gracilis Z.
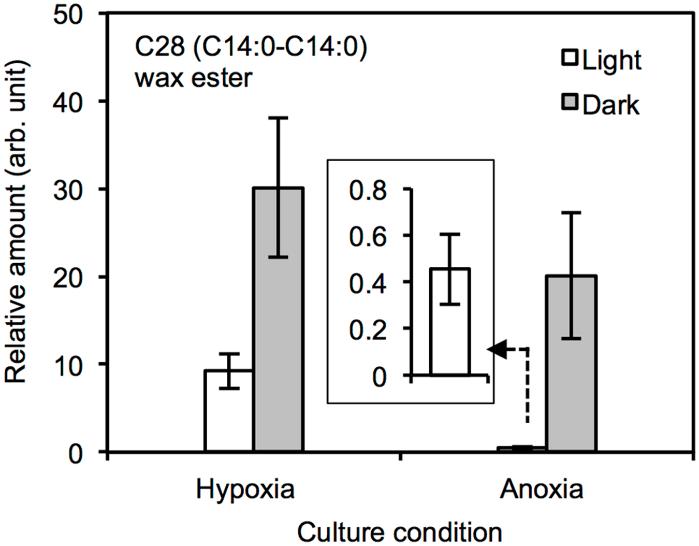 Inhibition of the wax ester fermentation in anoxia.
Inhibition of the wax ester fermentation in anoxia.
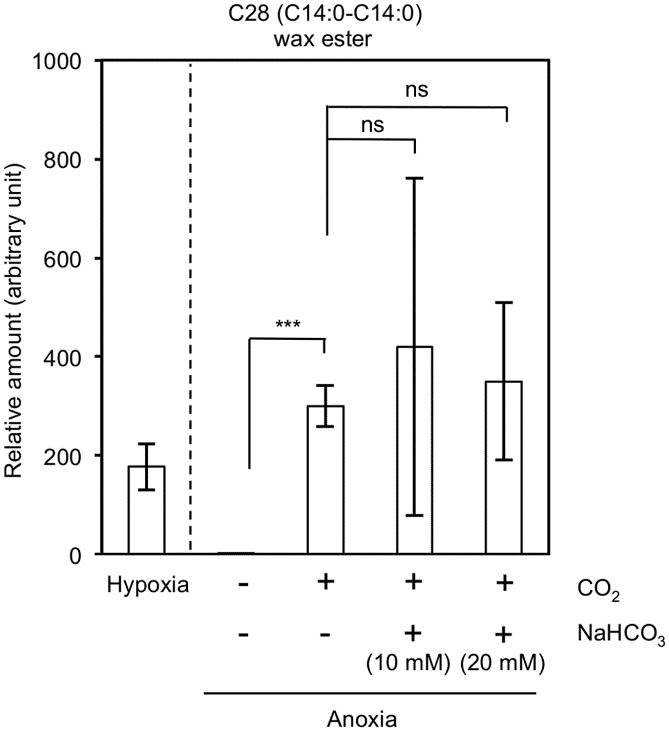 Recovery of the inhibited wax ester fermentation in anoxia by inorganic carbon sources.
Recovery of the inhibited wax ester fermentation in anoxia by inorganic carbon sources.
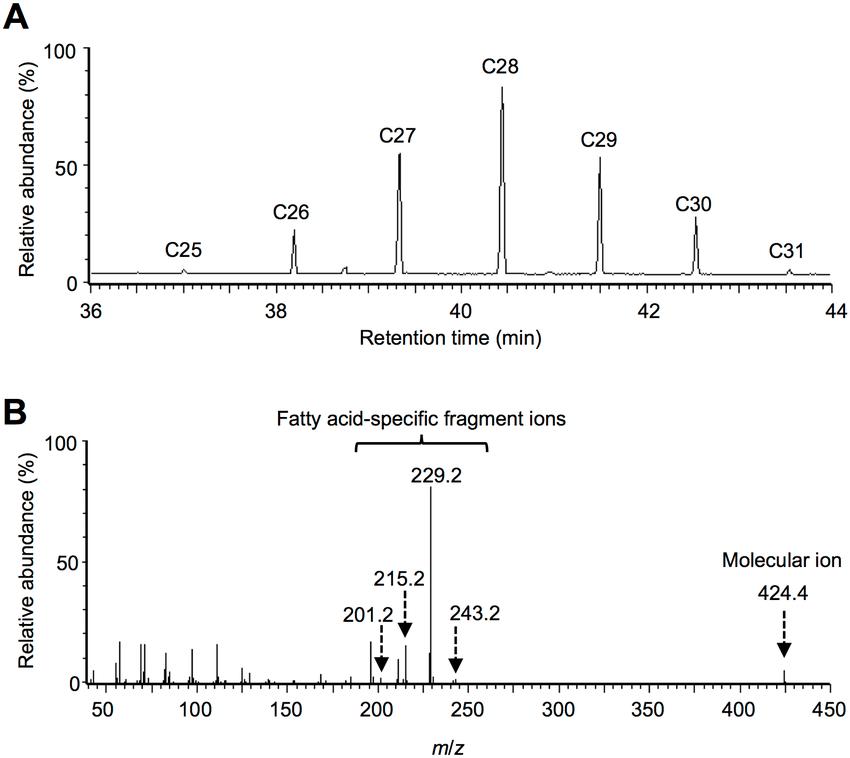 GC-MS analysis of the wax esters in E. gracilis Z under hypoxic-light conditions.
GC-MS analysis of the wax esters in E. gracilis Z under hypoxic-light conditions.
Reference
- Padermshoke, Adchara, et al. "Critical involvement of environmental carbon dioxide fixation to drive wax ester fermentation in Euglena." Plos one 11.9 (2016): e0162827.


 Inhibition of the wax ester fermentation in anoxia.
Inhibition of the wax ester fermentation in anoxia. Recovery of the inhibited wax ester fermentation in anoxia by inorganic carbon sources.
Recovery of the inhibited wax ester fermentation in anoxia by inorganic carbon sources. GC-MS analysis of the wax esters in E. gracilis Z under hypoxic-light conditions.
GC-MS analysis of the wax esters in E. gracilis Z under hypoxic-light conditions.