What are Homoserine Lactones?
Homoserine lactones (HSLs) are a class of signaling molecules, specifically belonging to the family of N-acyl homoserine lactones (AHLs). These compounds play a pivotal role in the process known as quorum sensing, which is essential for the regulation of gene expression in various bacterial populations. Quorum sensing enables bacteria to communicate and coordinate their behavior based on their density, influencing a myriad of physiological functions, including biofilm formation, virulence factor production, and bioluminescence.
Structurally, HSLs are characterized by a lactone ring and an acyl side chain, the length and composition of which can significantly affect their biological activity. Different bacterial species produce distinct HSLs, which allows for the specificity of signaling within microbial communities. Understanding the role and concentration of HSLs in microbial environments is crucial for various applications, including clinical diagnostics, agricultural practices, and environmental monitoring.
Homoserine Lactones Analysis Service by Creative Proteomics
Creative Proteomics provides specialized services for the analysis of homoserine lactones. The analysis of HSLs is essential for research in microbiology, pharmacology, and environmental science, given their role in mediating bacterial communication.
We offer customized projects tailored to the specific needs of researchers and industries. These projects may include:
- Quantitative Analysis: Determining the concentration of various HSLs in environmental samples, cell cultures, or microbial extracts.
- Qualitative Profiling: Identifying and characterizing the specific types of homoserine lactones present in a sample.
- Environmental Monitoring: Assessing the presence of HSLs in natural water bodies or soil to understand their impact on microbial ecology.
- Pathogen Detection: Investigating the role of HSLs in the virulence of pathogenic bacteria, aiding in the development of novel therapeutic approaches.
List of Homoserine Lactones We Can Detect
| Homoserine Lactone | Common Sources | Biological Role |
|---|
| N-Butyryl homoserine lactone (C4-HSL) | Agrobacterium tumefaciens, Pseudomonas aeruginosa | Regulates biofilm formation and virulence factors. |
| N-Hexanoyl homoserine lactone (C6-HSL) | Pseudomonas aeruginosa | Involved in quorum sensing and biofilm development. |
| N-Octanoyl homoserine lactone (C8-HSL) | Pseudomonas aeruginosa | Affects swarming and biofilm behaviors. |
| N-Dodecanoyl homoserine lactone (C12-HSL) | Pseudomonas aeruginosa, Vibrio fischeri | Modulates bioluminescence and pathogenicity. |
| N-Acyl homoserine lactone (AHL) | Various Gram-negative bacteria | General class; mediates diverse biological processes. |
| N-3-oxo-dodecanoyl homoserine lactone (3-oxo-C12-HSL) | Pseudomonas aeruginosa, Vibrio harveyi | Important for virulence and biofilm formation. |
| N-3-oxo-octanoyl homoserine lactone (3-oxo-C8-HSL) | Pseudomonas aeruginosa, Vibrio cholerae | Regulates virulence and biofilm development. |
| N-3-hydroxybutyryl homoserine lactone (3-OH-C4-HSL) | Various Gram-negative bacteria | Plays a role in biofilm maturation and maintenance. |
Analytical Techniques for Homoserine Lactones Analysis
Liquid Chromatography-Mass Spectrometry (LC-MS)
The primary instrument used for LC-MS analysis at Creative Proteomics is the Thermo Scientific Q Exactive Focus. This system offers high-resolution mass spectrometry capabilities, allowing for detailed analysis of HSLs.
LC-MS combines the physical separation capabilities of liquid chromatography with the mass analysis capabilities of mass spectrometry. It allows for:
- High Sensitivity: Detection of HSLs at low concentrations, which is essential for samples where HSLs may be present in trace amounts.
- Specificity: Differentiating between closely related compounds, providing precise identification of various HSLs.
Gas Chromatography-Mass Spectrometry (GC-MS)
For GC-MS, Creative Proteomics utilizes the Agilent 7890B GC System coupled with the Agilent 5977B MSD. This advanced system is designed for the analysis of volatile compounds and offers exceptional performance.
GC-MS is particularly beneficial for:
- Analyzing Volatile Compounds: Separating and quantifying HSLs that can easily evaporate, enhancing the understanding of their environmental behavior.
- Comprehensive Profiling: Identifying a broad range of HSLs within complex mixtures.
High-Performance Liquid Chromatography (HPLC)
The HPLC analysis is conducted using the Waters Acquity UPLC System. This instrument is known for its speed and efficiency in the separation of compounds.
HPLC is another technique used to analyze non-volatile HSLs. This method offers:
- Rapid Analysis: Efficient separation of HSLs, enabling quick turnaround times for results.
- Robust Data: Generating reliable quantitative data that researchers can utilize in their studies.
Sample Requirements for Homoserine Lactones Analysis
| Sample Type | Volume Required | Storage Conditions | Preservation Method |
|---|
| Environmental Water Samples | 50 mL | Refrigerated at 4°C | Filtered and preserved with sodium azide (if necessary) |
| Soil Samples | 25 g | Frozen at -20°C | Air-dried and stored in sealed containers |
| Bacterial Cultures | 10 mL | Refrigerated at 4°C | Stored in sterile conditions |
| Plant Extracts | 5 mL | Refrigerated at 4°C | Stabilized with appropriate preservatives |
| Biofilm Samples | 10 g | Frozen at -80°C | Preserved in cryoprotectants |
If you have any questions about our lipidomics services, please contact us.
Case Analysis of Quorum Sensing Molecules and Characterization of Pathogenic Bacteria Using Advanced Chromatographic Techniques
Background
Quorum sensing is a critical mechanism in bacteria, enabling population-wide coordination of activities such as bioluminescence, virulence, and biofilm formation. This process is facilitated through the production and detection of signaling molecules, such as acyl homoserine lactones (AHLs), furanosyl borate diesters, and oligopeptides. AHLs are particularly significant in Gram-negative bacteria and are synthesized via the Lux I enzyme family. These signaling molecules activate various cellular processes once a specific concentration threshold is reached, as demonstrated in species like Vibrio fischeri. Previous methods such as cell line bioreporters and thin-layer chromatography (TLC) have been used to identify these molecules, though their sensitivity was limited. With technological advancements, sophisticated methods such as high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS) allow for more accurate and sensitive detection of quorum sensing molecules.
Samples
Pathogenic bacteria belonging to the Vibrio genus were used for the study. Samples were collected from aquaculture sources, including moribund shrimp from a farm in Nagapattinam, Tamil Nadu. Additionally, pathogenic strains such as Vibrio cholerae, Vibrio parahemolyticus, and Vibrio fischeri were acquired from a research lab for comparison. The collected shrimp samples were homogenized, serially diluted, and cultured on TCBS agar for bacterial isolation. Biochemical tests were performed for identification based on Bergey's manual.
Techniques and Methods
Cell-Free Supernatant Preparation
Bacterial cultures were grown overnight in glycerol marine media, and the cells were centrifuged to separate the supernatant. This supernatant was filtered through 0.2 µm filters to remove residual cells. The cell-free culture supernatant was subjected to liquid-liquid extraction (LLE) using acidified ethyl acetate. The organic phase containing the extracellular molecules was collected, dried with nitrogen gas, and re-dissolved for subsequent analysis.
Thin-Layer Chromatography (TLC)
Extracted AHLs were separated on C18 silica TLC plates using a blend of organic solvents. The plates were visualized under UV light and by using chromic agents like potassium dichromate. Spots corresponding to AHLs were scraped off for further chemical extraction and analysis.
High-Performance Liquid Chromatography (HPLC)
Samples were injected into an HPLC system equipped with a C18 column, using acetonitrile and water with formic acid as the mobile phase. The flow rate and temperature were controlled precisely to optimize separation. Molecules were eluted at different rates based on their interactions with the column, allowing for the identification and quantification of various AHLs. The system's parameters, including pressure and solvent gradients, were carefully monitored to achieve optimal separation.
Gas Chromatography-Mass Spectrometry (GC-MS)
The extracted samples were further analyzed using GC-MS. Helium was used as the carrier gas, with the injector temperature set to 270°C. The column oven temperature was programmed to gradually increase to 300°C, ensuring the volatilization of the sample components. The mass spectrometer operated in SIM mode, targeting specific mass-to-charge ratios (m/z) for precise detection of AHLs. This method provided detailed identification and quantification of the signaling molecules.
Results
Collection and Identification of Isolates
The identification of various Vibrio isolates was conducted based on their growth characteristics on Thiosulfate-Citrate-Bile-Sucrose (TCBS) agar, followed by biochemical identification. Table 1 summarizes the growth and biochemical characteristics of the isolates.
Table 1: Growth and Biochemical Characteristics of Vibrio on TCBS Agar
| Characteristics | SPB1 | SPB2 | TC1 | TC2 | TC3 |
|---|
| Shape | Short comma | Short comma | Short comma | Short comma | Short comma |
| Gram Staining | -ve | -ve | -ve | -ve | -ve |
| Growth on TCBS Agar | Yellow colonies | Large yellow colonies | Flat yellow colonies | Blue to green centered colonies | Yellow-orange colonies |
| Identification | V. harveyi | V. alginolyticus | V. cholerae | V. alginolyticus | V. fischeri |
Thin Layer Chromatography (TLC)
TLC analysis of various bacterial metabolites demonstrated that both V. harveyi and V. alginolyticus produced AHLs at exponential rates. Comparison with the AHL standard derived from Chromobacterium violaceum confirmed the presence of similar AHL molecules among the isolates. The TLC results indicated that while V. cholerae contained three distinct communication molecules, the other isolates (V. harveyi, V. alginolyticus, V. parahemolyticus, and V. fischeri) revealed only one AHL each.
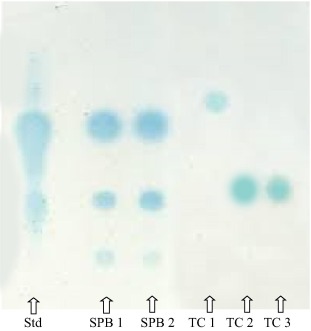 Thin layer chromatogram of various bacterial metabolites.
Thin layer chromatogram of various bacterial metabolites.
High-Performance Liquid Chromatography (HPLC)
The HPLC chromatogram for the predominant AHL-producing strain V. harveyi displayed seven peaks corresponding to various AHLs, with retention times as follows:
- C4-HSL: 3.224 min
- C6-HSL: 6.926 min
- 3-oxo-C8-HSL: 11.826 min
- C8-HSL: 16.012 min
- C10-HSL: 23.121 min
- C12-HSL: 28.103 min
- C14-HSL: 33.212 min
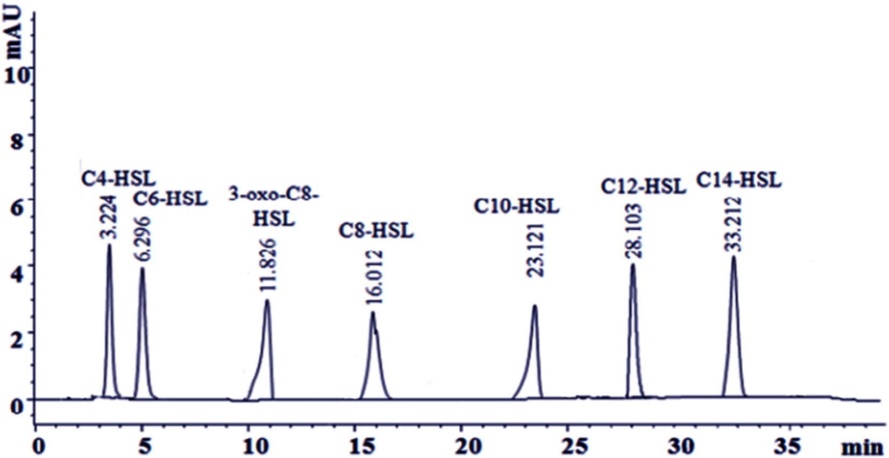 HPLC chromatogram of the predominant AHL producing strain V. harveyi.
HPLC chromatogram of the predominant AHL producing strain V. harveyi.
Gas Chromatography-Mass Spectrometry (GC-MS)
The GC-MS analysis revealed multiple peaks for isolated V. harveyi strains, confirming the presence of AHLs. Notably, a peak at a retention time of 215.4 confirmed the presence of AHL molecules, with each compound showing a characteristic molecular ion [M]+, typical for homoserine lactones.
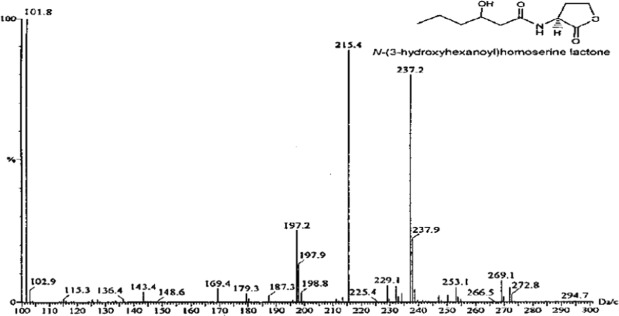 Chromatogram showing different peaks in the mass range of isolated V. harveyi strains.
Chromatogram showing different peaks in the mass range of isolated V. harveyi strains.
Reference
- Laj, Noha, et al. "Quorum-sensing molecules: Sampling, identification and characterization of N-acyl-homoserine lactone in Vibrio sp." Saudi Journal of Biological Sciences 29.4 (2022): 2733-2737.



 Thin layer chromatogram of various bacterial metabolites.
Thin layer chromatogram of various bacterial metabolites. HPLC chromatogram of the predominant AHL producing strain V. harveyi.
HPLC chromatogram of the predominant AHL producing strain V. harveyi. Chromatogram showing different peaks in the mass range of isolated V. harveyi strains.
Chromatogram showing different peaks in the mass range of isolated V. harveyi strains.