What are Polyunsaturated Fatty Acids?
Polyunsaturated fatty acids (PUFAs) are essential components of cellular membranes and are crucial for maintaining various physiological functions. Unlike saturated fats, which have no double bonds between carbon atoms, PUFAs possess two or more double bonds in their carbon chain. This structural characteristic imparts unique properties and functions to PUFAs, distinguishing them from other types of fatty acids.
PUFAs are broadly classified into two main categories based on their chemical structure:
- Omega-3 Fatty Acids: Includes eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and alpha-linolenic acid (ALA). They are known for their anti-inflammatory properties and benefits for heart, brain, and eye health.
- Omega-6 Fatty Acids: Includes linoleic acid (LA) and arachidonic acid (AA). Vital for cellular functions and growth but can contribute to inflammation if not balanced with omega-3s.
Creative Proteomics offers a comprehensive suite of analytical services dedicated to the precise measurement and characterization of polyunsaturated fatty acids. These services cater to a wide range of applications, including clinical research, nutritional studies, and pharmaceutical development.
Polyunsaturated Fatty Acids Analysis Service by Creative Proteomics
Polyunsaturated Fatty Acids Quantitative Analysis: Measuring the concentration of specific PUFAs in biological samples, such as plasma, tissues, or dietary supplements. Accurate quantification helps in understanding the metabolic status and dietary intake of these essential fats.
Polyunsaturated Fatty Acids Profile Analysis: Profiling the overall composition of PUFAs in various samples to assess the balance and distribution of omega-3 and omega-6 fatty acids. This is particularly useful in nutritional studies and health assessments.
Polyunsaturated Fatty Acids Metabolite Analysis: Investigating the metabolic pathways of PUFAs and their derivatives to understand their biological effects and potential therapeutic applications.
Quality Control: Ensuring the purity and consistency of PUFA-containing products through rigorous quality control measures. This is essential for the development of supplements and pharmaceuticals.
List of Polyunsaturated Fatty Acids We Can Analyze
| Fatty Acid | Type | Description |
|---|
| Alpha-Linolenic Acid (ALA) | Omega-3 | Essential fatty acid found in plant oils, important for cardiovascular health. |
| Eicosapentaenoic Acid (EPA) | Omega-3 | Found in fish oils, crucial for anti-inflammatory effects and heart health. |
| Docosahexaenoic Acid (DHA) | Omega-3 | Major component of brain and retinal tissues, supports cognitive and visual functions. |
| Docosapentaenoic Acid (DPA) | Omega-3 | Less studied than EPA and DHA, contributes to cardiovascular health. |
| Stearidonic Acid (SDA) | Omega-3 | Found in some plant oils, a precursor to EPA. |
| Linoleic Acid (LA) | Omega-6 | Essential fatty acid found in vegetable oils, involved in cellular functions and growth. |
| Gamma-Linolenic Acid (GLA) | Omega-6 | Found in certain oils, has anti-inflammatory properties. |
| Dihomo-Gamma-Linolenic Acid (DGLA) | Omega-6 | Metabolite of GLA, involved in inflammation regulation. |
| Arachidonic Acid (AA) | Omega-6 | Important for inflammatory responses and cell membrane structure. |
| Eicosadienoic Acid (EDA) | Omega-6 | Intermediate in the metabolism of linoleic acid. |
| Adrenic Acid (AdA) | Omega-6 | Found in adrenal glands, involved in inflammatory responses and cell signaling. |
| Homo-Gamma-Linolenic Acid (HGLA) | Omega-6 | Precursor to DGLA, involved in inflammatory and immune responses. |
Analytical Techniques for Polyunsaturated Fatty Acids Analysis
Gas Chromatography with Flame Ionization Detection (GC-FID): This technique involves converting fatty acids into fatty acid methyl esters (FAMEs), which are then vaporized and separated in a chromatographic column. The flame ionization detector quantifies the separated FAMEs based on the electrical current produced by ionized compounds, providing precise quantification of individual PUFAs and a comprehensive fatty acid profile.
Liquid Chromatography-Mass Spectrometry (LC-MS): Fatty acids are separated using liquid chromatography and then identified and quantified by mass spectrometry, which measures the mass-to-charge ratio of the compounds. This method offers high sensitivity and specificity, making it suitable for analyzing PUFAs in complex biological samples.
High-Performance Liquid Chromatography (HPLC): Fatty acids are separated based on their interactions with the chromatographic column. Various detectors, such as UV or fluorescence detectors, are employed to identify and quantify the PUFAs. HPLC is effective for profiling and quantifying fatty acids and is often used in conjunction with other techniques to enhance accuracy.
Sample Requirements for Polyunsaturated Fatty Acids Analysis
| Sample Type | Preparation Requirements | Storage Conditions | Sample Volume/Weight |
|---|
| Plasma | Centrifuge to separate plasma from blood cells. | Store at -80°C for long-term storage. | 1-2 mL per sample. |
| Tissues | Homogenize and extract lipids using appropriate solvents. | Store at -80°C to prevent degradation. | 50-100 mg of tissue per sample. |
| Dietary Supplements | Homogenize and dissolve in suitable solvents for analysis. | Store as per manufacturer's guidelines. | 100-500 mg of powder or liquid. |
| Cell Cultures | Harvest cells and extract lipids. | Store at -80°C for accurate results. | 1-2 million cells or 1-2 mL of cell culture. |
If you have any questions about our lipidomics services, please contact us.
Case Adrenic Acid as a Key Regulator in Inflammation Resolution
Background
Inflammation is a critical defense response to tissue damage, typically well-regulated but can lead to chronic diseases like atherosclerosis and cancer if unresolved. Polyunsaturated fatty acids (PUFAs) are key regulators of inflammation. Traditionally, n-6 PUFAs, such as arachidonic acid (AA), are seen as proinflammatory, while n-3 PUFAs, like eicosapentaenoic acid (EPA), are considered anti-inflammatory.
Recent findings challenge this strict classification, showing that n-6 PUFAs can also have anti-inflammatory effects. Clinical trials of n-3 PUFA supplementation have produced mixed results, suggesting that the roles of n-6 and n-3 PUFAs in inflammation are more complex than previously thought.
This study further explores these roles by analyzing PUFA dynamics in a model of self-resolving peritonitis, discovering the accumulation of the n-6 PUFA adrenic acid (AdA) during inflammation resolution, leading to a deeper investigation of its biological function.
Materials & Methods
Cell Isolation and Culture
Human neutrophils were isolated from EDTA blood via dextran sedimentation and Ficoll gradient centrifugation. PBMCs were isolated similarly, and monocytes were separated and differentiated into macrophages using GM-CSF.
Cell Culture for Lipid Analysis
Neutrophils and macrophages were incubated with adrenic acid (AdA) and stimulated with calcium ionophore. Cells and supernatants were prepared for lipidomic analysis and stored at −80°C.
Calcium Flux Assay
Leukocytes labeled with Indo-1 AM were preincubated with AdA and analyzed by flow cytometry after stimulation with calcium ionophore.
Phagocytosis and Efferocytosis Assays
THP-1 cells and macrophages were incubated with AdA and analyzed for phagocytosis of FITC-labeled zymosan and efferocytosis of apoptotic HL-60 cells using flow cytometry.
Migration Assay
Neutrophil migration towards supernatants from AdA-treated cells was quantified by flow cytometry.
In Vivo Peritonitis Model
Peritonitis was induced in mice by zymosan A injection, followed by AdA treatment. Peritoneal lavage was collected for cell and lipid mediator analysis.
K/BxN Serum-Transferred Arthritis Model
Arthritis was induced in mice using K/BxN serum, with daily AdA treatment. Clinical scores and anti-G6PI antibody levels were measured.
Lipidomic Analysis
Lipid mediators were analyzed using LC-MS/MS and GC-MS, employing internal standards for quantification.
Statistical Analysis
Data were analyzed using appropriate statistical tests, including t-tests and ANOVA.
Results
Accumulation of AdA During Inflammation Resolution:
In a zymosan A-induced murine peritonitis model, a significant increase in cellular Adrenic Acid (AdA) was observed during the resolution phase of inflammation, indicating a potential pro-resolving role for this n-6 polyunsaturated fatty acid (PUFA). This accumulation varied across different PUFAs, with AdA showing an 80-fold increase, suggesting its involvement in promoting the resolution of inflammation.
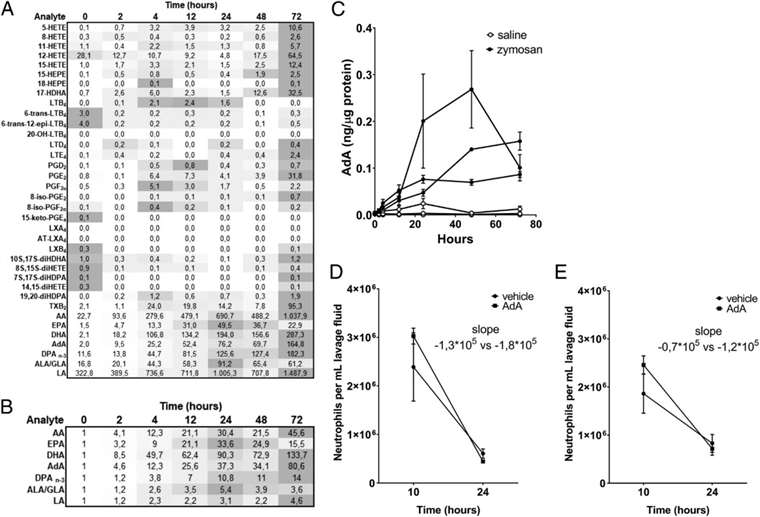 Heatmap and fold change in PUFA levels during zymosan A-induced peritonitis. Mice were treated with AdA at different times, showing enhanced neutrophil clearance.
Heatmap and fold change in PUFA levels during zymosan A-induced peritonitis. Mice were treated with AdA at different times, showing enhanced neutrophil clearance.
AdA Inhibits LTB4 Production in Neutrophils:
AdA exposure significantly reduced Leukotriene B4 (LTB4) production in neutrophils upon stimulation, while its effect on macrophages varied among donors. This inhibition was associated with decreased arachidonic acid (AA) levels, suggesting AdA's role in attenuating the LTB4 synthesis pathway without affecting cell viability or intracellular calcium flux.
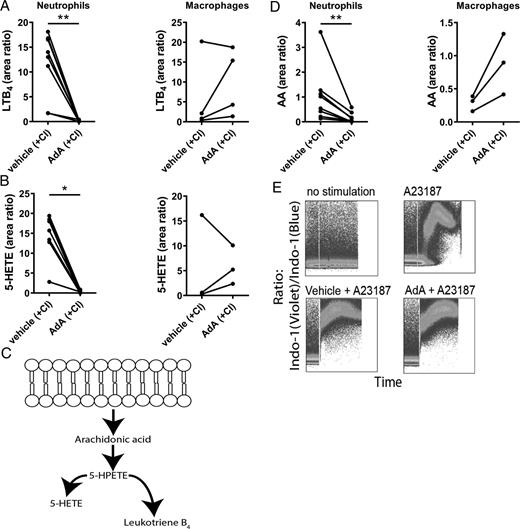 Inhibition of LTB4 production by AdA in neutrophils. Reduction in LTB4 and 5-HETE levels upon AdA treatment in human neutrophils and macrophages.
Inhibition of LTB4 production by AdA in neutrophils. Reduction in LTB4 and 5-HETE levels upon AdA treatment in human neutrophils and macrophages.
Reduced Chemoattractant Capacity of AdA-Exposed Neutrophils:
AdA-treated neutrophils exhibited diminished chemoattractant ability, significantly reducing their migration toward LTB4 and supernatants from stimulated neutrophils, indicating a potential reduction in inflammation propagation.
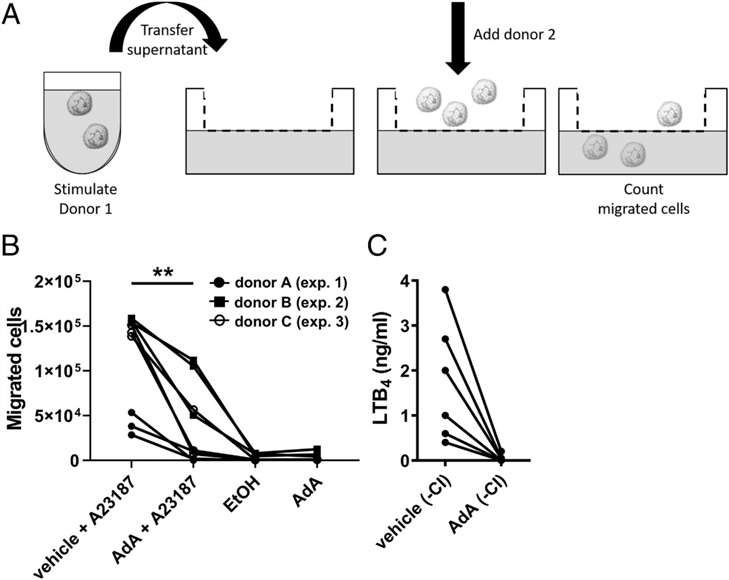 Impaired neutrophil migration toward supernatants from AdA-treated neutrophils.
Impaired neutrophil migration toward supernatants from AdA-treated neutrophils.
Enhanced Macrophage Phagocytosis by AdA:
AdA significantly increased the phagocytic activity of macrophages, including both zymosan particles and apoptotic cells, suggesting an enhanced resolution of inflammation.
AdA Uptake and Lipid Reservoirs in Neutrophils:
AdA was found to be internalized by neutrophils and stored in various lipid reservoirs, primarily cholesteryl esters, and to a lesser extent in phosphatidylcholines (PCs), diacylglycerides (DAGs), and triglycerides (TAGs). No significant rearrangement of AA in these lipid compartments was observed, suggesting that AdA modulates AA release by other mechanisms.
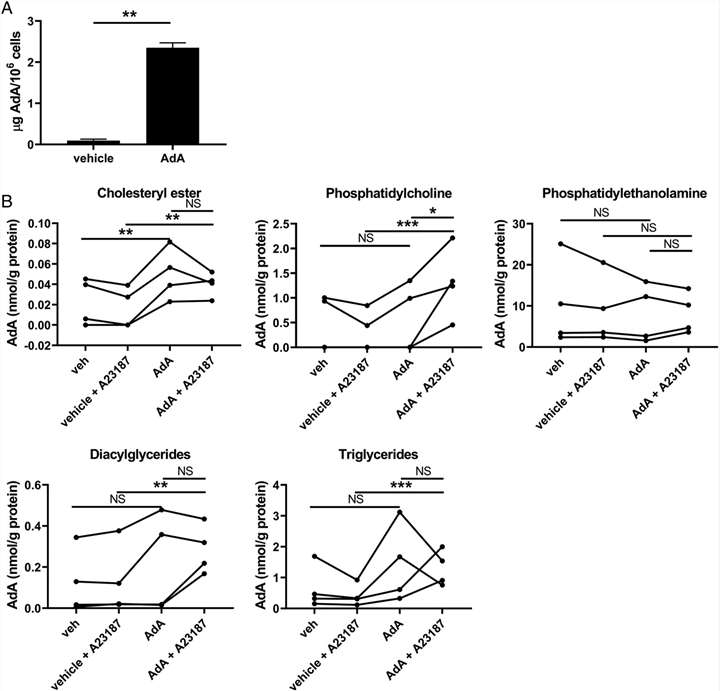 AdA accumulation in neutrophil lipid reservoirs post-treatment.
AdA accumulation in neutrophil lipid reservoirs post-treatment.
AdA Reduces Severity of Neutrophil-Mediated Experimental Arthritis
In an experimental arthritis model, AdA treatment led to a significant reduction in arthritis severity, correlated with lower levels of anti-G6PI antibodies, indicating its potential therapeutic effects in inflammatory diseases.



 Heatmap and fold change in PUFA levels during zymosan A-induced peritonitis. Mice were treated with AdA at different times, showing enhanced neutrophil clearance.
Heatmap and fold change in PUFA levels during zymosan A-induced peritonitis. Mice were treated with AdA at different times, showing enhanced neutrophil clearance. Inhibition of LTB4 production by AdA in neutrophils. Reduction in LTB4 and 5-HETE levels upon AdA treatment in human neutrophils and macrophages.
Inhibition of LTB4 production by AdA in neutrophils. Reduction in LTB4 and 5-HETE levels upon AdA treatment in human neutrophils and macrophages. Impaired neutrophil migration toward supernatants from AdA-treated neutrophils.
Impaired neutrophil migration toward supernatants from AdA-treated neutrophils. AdA accumulation in neutrophil lipid reservoirs post-treatment.
AdA accumulation in neutrophil lipid reservoirs post-treatment.