Adverse Drug Reactions Biomarker
Adverse drug reactions (ADRs), untoward outcomes of therapeutic drugs, occur along with their main therapeutic effect, thereby raise safety concerns in drug development and disease treatment. In severe cases of ADRs, both upcoming drugs and approved drugs are withdrawn. Therefore, biomarkers for ADR susceptibility, onset, severity, and recovery, have been a major focus of research in pharmacology and toxicology. The diverse functions of lipids in the maintenance of general health and prevention of diseases have been elucidated. Lipids are well-known structural components of the membranes of cells, organelles, and vesicles. They act as a source of energy and play an important role in cell signaling pathways. Owing to these properties, lipids are considered suitable targets for biomarker development. FAs, GLs, GPs, SLs, and STs are relatively abundant in the mammalian body and are the major targets of lipidomics used to identify biomarker candidates for diseases and organ toxicities, including ADRs.
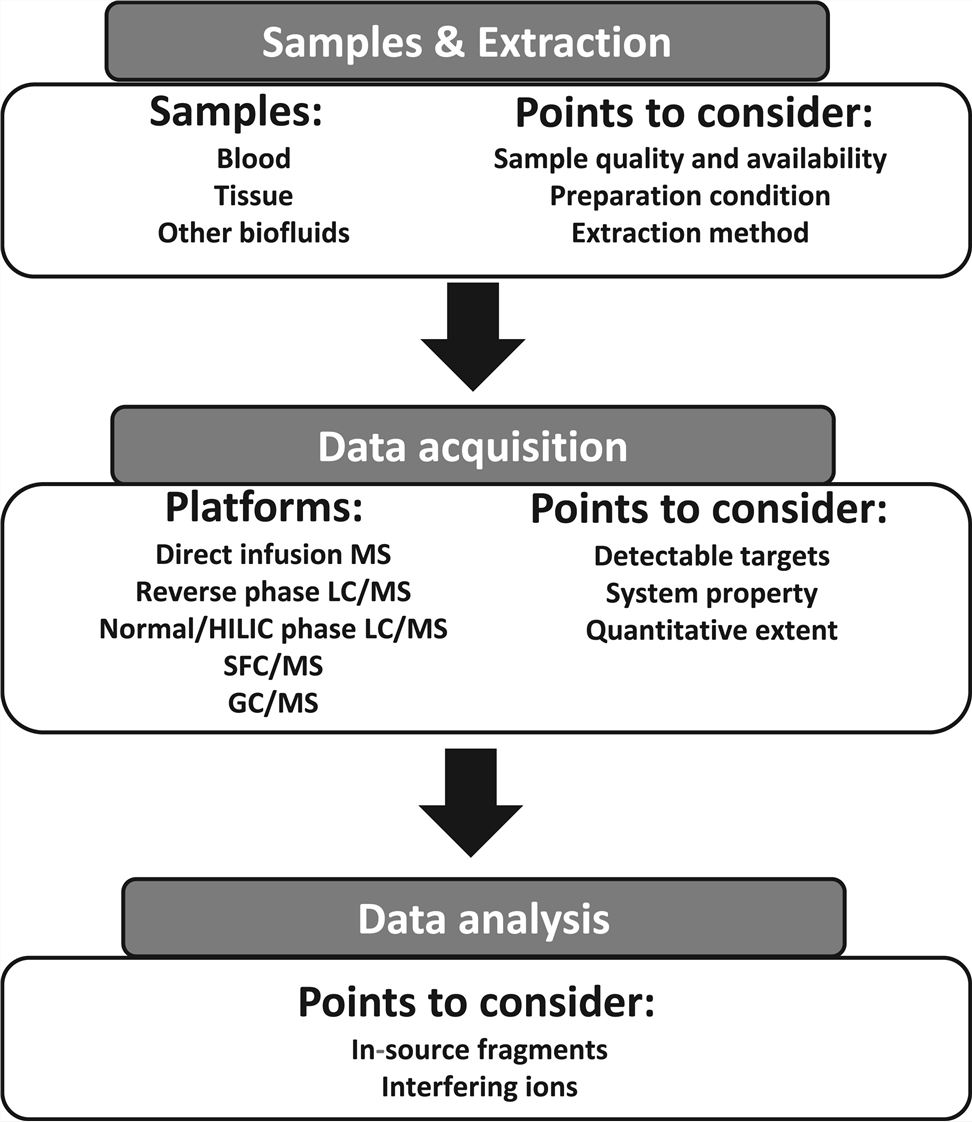 Fig1. Schematic workflow and points to consider in ADR lipidomics (Saito, Kosuke, 2020)
Fig1. Schematic workflow and points to consider in ADR lipidomics (Saito, Kosuke, 2020)
Case
- Drug-induced-ADRs in the liver
As lipidomics involves lipids, its application to ADRs is primarily applied to organs closely related to lipid metabolism, especially the liver. Drug-induced liver lipid disorders, such as phospholipidosis and steatosis, are directly associated with lipid metabolism and classified as ADRs in the liver. Drug-induced liver phospholipidosis have been reported to induce inflammation and fibrosis in the liver.
A lipidomics analysis using three tricyclic antidepressant-induced phospholipidosis models has demonstrated that decreased plasma lysophosphatidylcholines (LPCs) and increased monoglycosylceramides (CerG1s) are common markers for liver phospholipidosis. Furthermore, another study using a tamoxifen-induced liver phospholipidosis model demonstrated that decreased plasma LPC and arachidonic acid-containing PC (AA-PC) is associated with liver phospholipidosis.
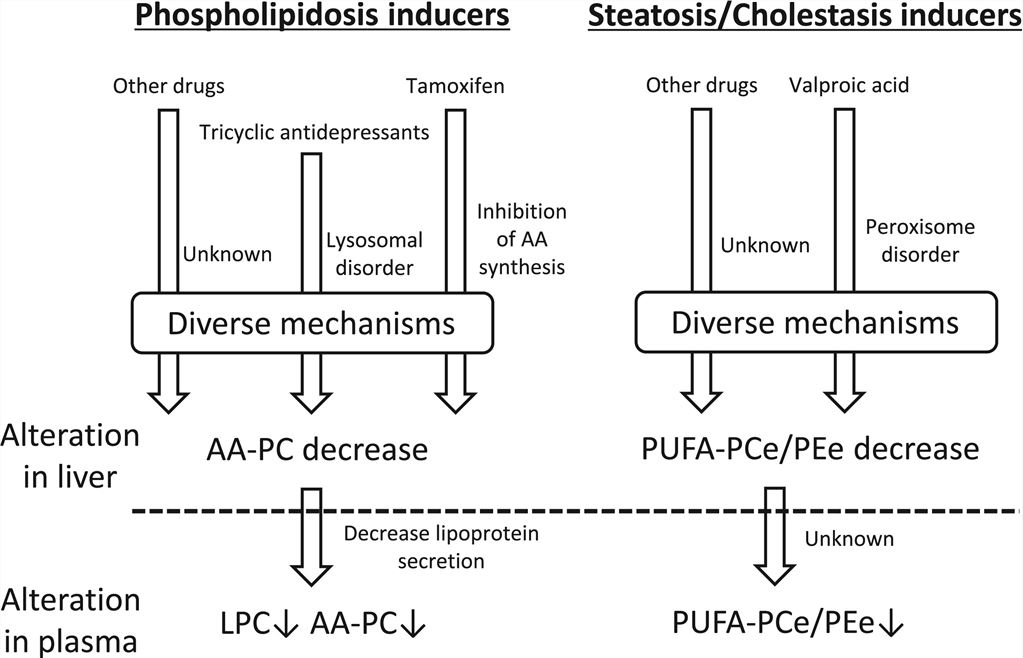 Fig2. Putative mechanisms underlying blood markers for lipid disorders in the liver biomarkers (Saito, Kosuke, 2020)
Fig2. Putative mechanisms underlying blood markers for lipid disorders in the liver biomarkers (Saito, Kosuke, 2020)
- Drug-induced ADRs in other tissues
Lipidomics analysis using tissues is useful for addressing pathophysiological changes in the tissues. Tacrine-induced brain mitochondrial toxicity involves remodeling the brain lipid status with increased levels of PEs but decreased levels of cardiolipins (CLs), which are abundant in the mitochondria. Additionally, cisplatin treatment upregulated Cers and CerG1s in the kidney, suggesting the involvement of ceramide-induced kidney cell death. The association of insulin resistance with decreased Cers has also been demonstrated in muscles, suggesting altered sensitivity to insulin signal.
With decades of operational experience and technology platform, Creative Proteomics provides reliable, rapid, and cost-effective untargeted lipidomics and targeted lipidomics services based on GC/LC-MS and shot-gun methods for adverse drug reactions biomarker study.
Available Services
How we work in 7 easy steps

Creative Proteomics provides a perfect lipidomics platform for adverse drug reactions biomarker study. If you have any special needs for adverse drug reactions biomarker analysis, please contact us. Let us know what you need and we will accommodate you. We look forward to working with you in the future.
Reference:
- Saito, Kosuke. Application of comprehensive lipidomics to biomarker research on adverse drug reactions. Drug Metabolism and Pharmacokinetics. 2020: 100377.
* Our services can only be used for research purposes and Not for clinical use.
Applications:


 Fig1. Schematic workflow and points to consider in ADR lipidomics (Saito, Kosuke, 2020)
Fig1. Schematic workflow and points to consider in ADR lipidomics (Saito, Kosuke, 2020) Fig2. Putative mechanisms underlying blood markers for lipid disorders in the liver biomarkers (Saito, Kosuke, 2020)
Fig2. Putative mechanisms underlying blood markers for lipid disorders in the liver biomarkers (Saito, Kosuke, 2020)